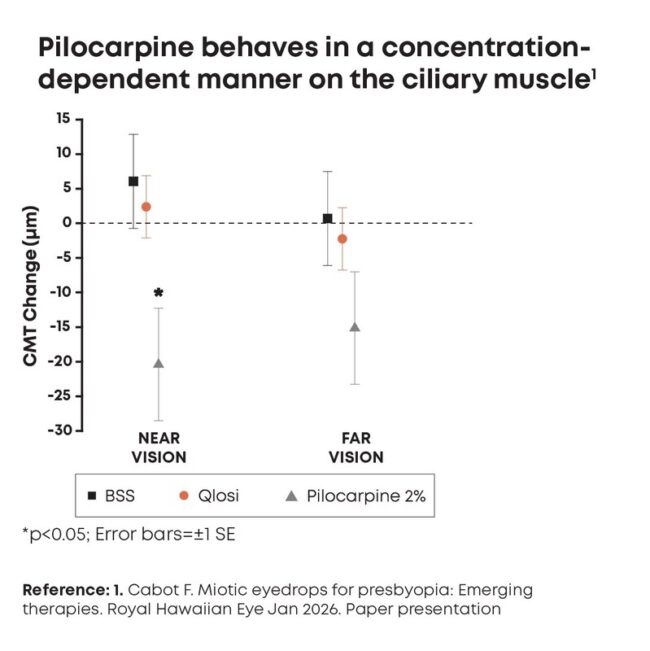
Qlosi reduces pupil diameter with no significant impact on ciliary muscle
Qlosi (Orasis Pharmaceuticals), low dose (0.4%) pilocarpine, reduces pupil diameter with no significant impact on ciliary muscle.
Bascom Palmer Eye Institute in Miami, shared data that compared the impact of Qlosi, balanced salt solution, and 2% pilocarpine on the ciliary muscle thickness. Only the high dose 2% pilocarpine solution showed statistically significant change in ciliary muscle thickness, while Qlosi showed no significant changes. This highlights the safety profile of Qlosi compared to other high dose myotics in the market.
Dr. Cabot also said in a press release from Orasis: “These findings are very encouraging, supporting that pilocarpine 0.4% is pupil selective and behaves in a concentration-dependent manner,
reinforcing that low-concentration options may be particularly meaningful for patients considering a presbyopia therapy that balances both efficacy and safety.”